Human Papilloma Virus (HPV)
Human Papilloma Virus (HPV)
WHY TEST?
Consider these burden of disease facts. Worldwide, in 2018, the 4th most common cause of new cancer cases in women, as well as the 4th most common cause of cancer death for women is tied to HPV.1,2
In 2020, According to the American Cancer Society Journal (ACSJ), cervical cancer was again named the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death in women.
An estimated 604,000 women were diagnosed with cervical cancer worldwide and about 342,000 women died from the disease in 2020. In addition, cervical cancer is the most commonly diagnosed cancer in 23 countries.3
Within the United States, cervical cancer death rates are 2-fold higher among women residing in high-poverty versus low-poverty areas.3
- Cronin KA, Lake AJ, & Scott S, et al. Cancer 2018 Jul 1;124(13):2785–2800 Centers for Disease Control. Preventing Cervical Cancer in the 21st Century. Accessible Version:20190125-presentation-cervical-cancer-H.pdf
- www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-4th-edition.pdf gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi:10.3322/caac.21660. (American Cancer Society https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660)
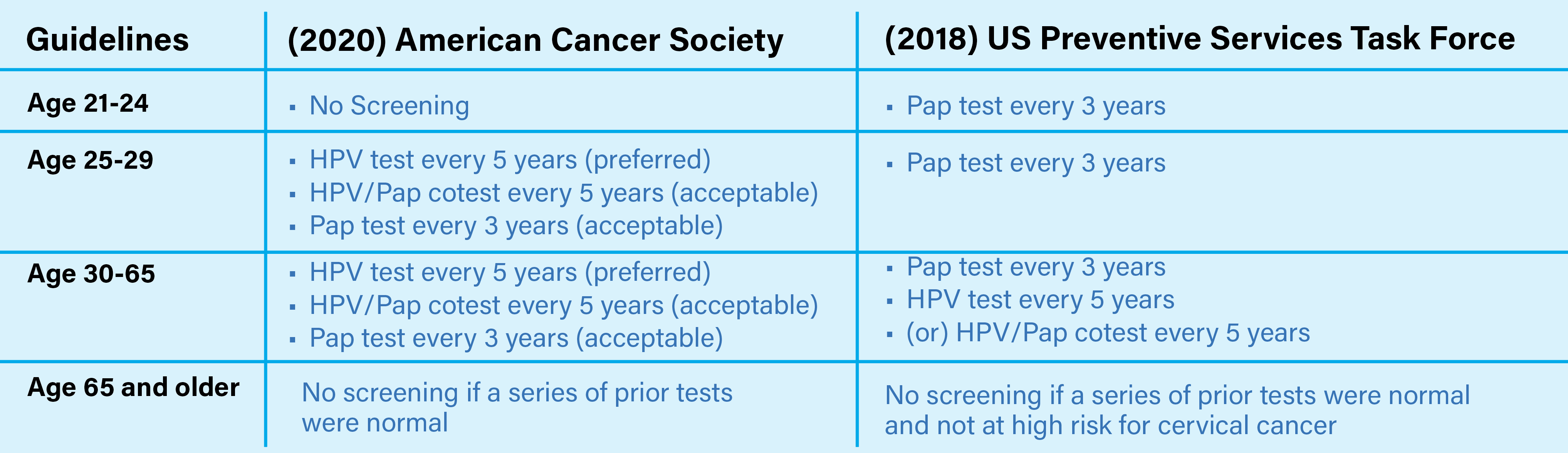
WHO TO TEST?
High-quality screening programs are important to prevent cervical cancer among unvaccinated women and for oncogenic subtypes not covered by the vaccine. In the absence of effective screening, there have been rapid increases in premature cervical cancer mortality.1
Using an HPV DNA NAAT as the primary screening test prevents more cervical cancers and saves more lives than using visual inspection with acetic acid (VIA) or cytology (conventional Pap smear and liquid-based cytology) as the primary screening test.2
Accumulated evidence supports the use of HPV-based tests for the detection of precancerous lesions as a preferred test for primary screening. According to the World Health Organization (WHO) continuing screening is crucial and existing programs with quality-assured cytology or VIA as primary screening tests should be continued until access to HPV NAATs screening, like the SimplyHPV™ test, are able to replace them.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi:10.3322/caac.21660. (American Cancer Society https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660)
- Human papillomavirus (HPV) nucleic acid amplification tests (NAATs) to screen for cervical pre-cancer lesions and prevent cervical cancer: policy brief ISBN 978-92-4-004524-8 (electronic) ISBN 978-92-4-004525-5 (print)
FAQ’s
Frequently Asked Questions